Tacoma-based Revalesio receives key designation for ALS drug
Revalesio’s RNS60 achieves orphan drug status
There’s new hope for people suffering from amyotrophic lateral sclerosis (ALS)—a promising drug treatment developed by Tacoma-based biomedical company Revalesio.
The U.S. Food and Drug Administration recently granted orphan drug designation for Revalesio’s investigational drug RNS60 used to treat ALS, also known as Lou Gehrig’s disease. The progressive disease causes nerve cells to deteriorate, and leads to total paralysis and death. No available treatment has been shown to stop, reverse or prevent ALS, which affects around 15,000 people in the U.S.

The orphan drug designation program provides incentives for pharmaceutical companies to develop products to treat rare diseases and disorders that affect fewer than 200,000 people in the U.S.
ALS has come to the forefront in recent years. Actor and writer Sam Shepard recently succumbed to the disease. In 2014, the Ice Bucket Challenge became a viral phenomenon and gave a huge boost to ALS awareness and fundraising efforts. Millions of dollars raised from the campaign are now helping to fund major breakthroughs like RNS60.
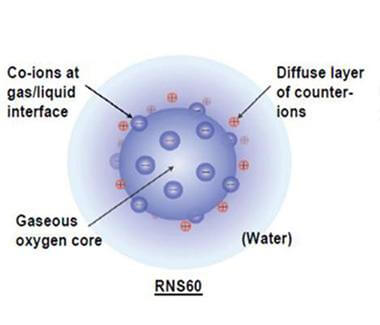
In simple terms, RNS60 consists of saline and oxygen modified using Revalesio’s patented technology to create oxygen nanobubbles. Preclinical studies have shown RNS60 significantly reduces inflammation that can accelerate ALS. The drug is being evaluated in a pilot study at Massachusetts General Hospital (MGH). At clinical centers in Italy, as well as at MGH, a larger study is evaluating the effect of RNS60 on biomarkers of the disease.
“With orphan status, we will have increased access to the FDA to help facilitate RNS60’s drug development plan and, hopefully, bring RNS60 to patients who are suffering from ALS,” said Greg Archambeau, president of Revalesio, which has partnered with global leaders in biomedical research to develop the drug.
Revalesio and its collaborators aim to develop additional uses of RNS60 as treatment for Multiple Sclerosis, Alzheimer’s and Parkinson’s disease, asthma and other inflammatory diseases.
On a lighter note, Revalesio’s oxygen-nanobubble technology is also used in a popular beverage designed to improve muscle recovery after exercise. Reliant Beverage Company, a wholly owned subsidiary of Revalesio, produces Recovery Water™, a favorite of athletes, from world-class pros to weekend warriors.
For more information, visit revalesio.com.